NET cancer — or neuroendocrine tumor cancer — isn’t well known. It isn’t branded by the color pink and it doesn’t have a month dedicated to raising awareness. It isn’t associated with a controversial habit such as smoking that ignites passion on both sides of the conversation.
When Steve Jobs and Aretha Franklin passed away of NET cancer, the media erroneously reported it as an entirely different type of cancer. And when someone receives a diagnosis, the first question that often comes to mind is, “What is NET cancer?”
As with many things in life, the squeaky wheel gets the grease. Over the years, common cancers have garnered more support and resources — and therefore many more treatment options.
But in 2018, NET cancer finally had its moment in the spotlight.
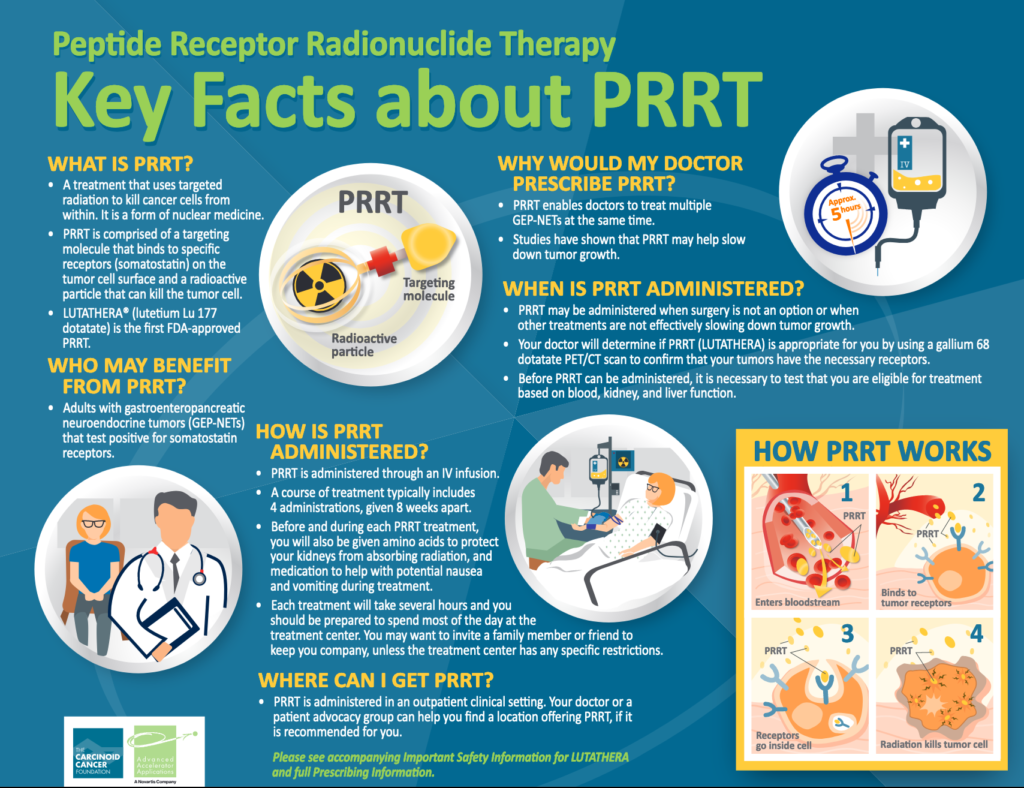
That year, Lutathera (lutetium Lu177 dotatate) became the first peptide receptor radionuclide therapy (PRRT) to receive U.S. Food and Drug Administration (FDA) approval for the treatment of adult patients with a certain type of NET cancer — gastroenteropancreatic NETs (GEP-NETs) that are positive for the hormone receptor somatostatin, including GEP-NETs in the foregut, midgut, and hindgut. The therapy was approved in 2017 by the European Commission (EC). Since then, more than 9,000 U.S. and European patients have been treated with Lutathera, and it remains the only approved PRRT on the market.
PRRT and Lutathera
PRRT, also known as a radioligand therapy, is a class of targeted molecular therapies composed of a peptide linked to a radioactive material (radionuclide). The peptide is highly specific for certain receptors on tumor cells. It attaches to these receptors in a “lock and key” mechanism, which triggers the delivery of the radionuclide that kills the tumor cells. As a treatment for NET cancer, PRRT has been used as an experimental therapy in several countries in Europe since 1996. A number of radionuclides have been tested over the years, but lutetium 177 has been one of the most effective.
Lutathera is a PRRT. Its peptide is a somatostatin analog that specifically recognizes and binds to cells with somatostatin receptors. The radionuclide linked to it is lutetium 177. Not all patients with NET cancer have somatostatin receptors on the tumor cell surfaces, so only those who are confirmed to have somatostatin receptors are eligible for Lutathera treatment.
The Evolving Treatment Landscape for NET Cancer
Treatments are not “one size fits all” because patients with NET cancer are not a homogenous group. Unlike organ-specific cancers, NET cancer originates in neuroendocrine cells, which are found throughout the body, such as in the small intestine, colon, pancreas, lung, etc.
Each type of NET cancer is unique and not every patient responds to treatment in the same way. For example, some patients experience symptoms of carcinoid syndrome, such as flushing, watery diarrhea, fatigue, etc., while others may have no symptoms at all. Some have tumors that express somatostatin while others do not. Some respond well to a specific treatment while others do not.
This is why having a range of treatment options is so important. Drug approvals are generally based on cumulative patient data gathered and analyzed from controlled clinical trials. According to the clinicaltrials.gov database, there are 742 global clinical trials actively recruiting patients with NET cancer. More than 400 NET cancer clinical trials have been completed and have reported data.
Lutathera is one of the most exciting advances in NET cancer treatment. It is a first-in-class therapy that has sparked enthusiasm within the medical and patient communities and has inspired increased interest in NET cancer research. I look forward to seeing the next wave of innovative treatment options for NET cancer!
- For more information about the clinical trial that supported Lutathera’s approval, read the original 2017 manuscript of the NETTER-1 clinical trial results that was published in the New England Journal of Medicine. The final overall survival data in the NETTER-1 trial was published in 2021 and is discussed in this ASCO Post article.
- For more information about NET cancer clinical trials, check out the resources compiled by the Los Angeles Carcinoid Neuroendocrine Tumor Society (LACNETS).
- For information about current clinical practice guidelines, take a look at the ESMO Clinical Practice Guidelines for Gastroenteropancreatic Neuroendocrine Neoplasms.

Leave a Reply